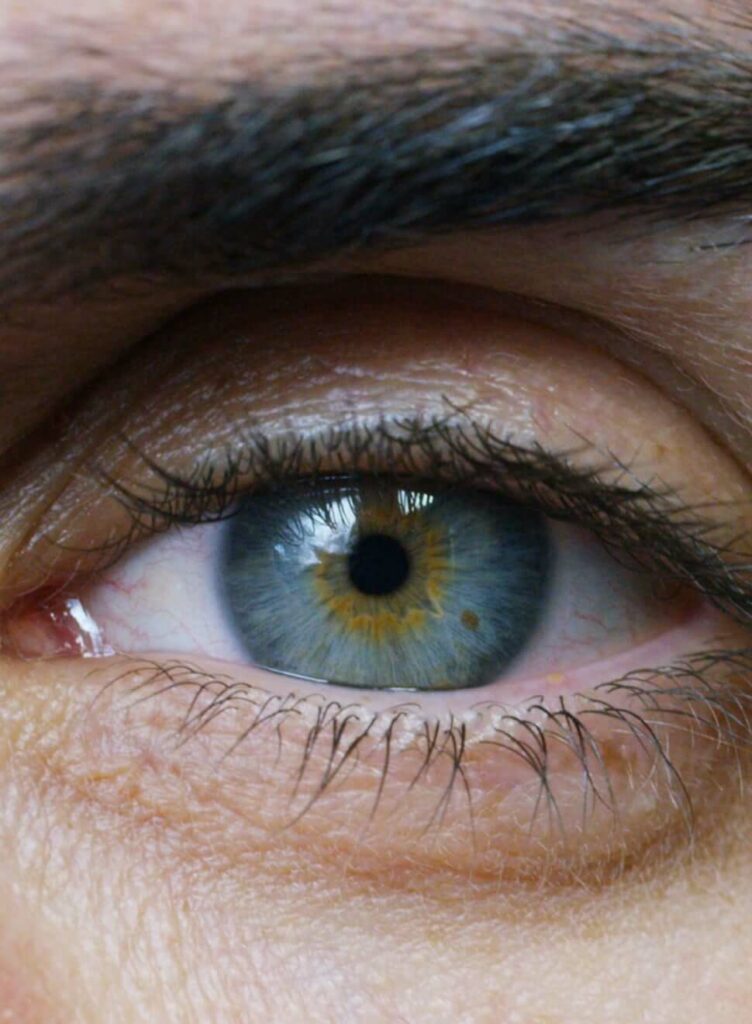
What is LHON?
Leber’s hereditary optic neuropathy (LHON) is a rare, inherited form of mitochondrial disease resulting in rapid central vision loss.Footnote1,Footnote2 The treatment of LHON remains challenging, and the visual prognosis is quite poor.Footnote1 German ophthalmologist Theodore Leber defined it as a clinical condition in 1871.Footnote2
LHON was the first human condition linked to a point mutation in the mitochondrial DNA (mtDNA). These mutations affect subunit genes of complex I.Footnote3 LHON presents with subacute, simultaneous or sequential, bilateral painless loss of central vision due to selective degeneration of the retinal ganglion cells (RGCs) and their axons.Footnote2 These cells are particularly vulnerable to mitochondrial dysfunction leading to optic nerve atrophy and loss of central vision.Footnote2 Most patients with LHON end up legally blind.Footnote1
-
LHON is a mitochondrial disorder that results from point mutations in the mtDNA that encode components of respiratory complex 1 (See figure below).Footnote4 Approximately 90% of the LHON cases come from three primary mtDNA mutations (m.3460G>A in MT-ND1, m.11778G>A in MT-ND4, and m.14484T>C in MT-ND6) that are maternally inherited.Footnote4,Footnote5
Although an mtDNA pathogenic mutation is an important factor, other mitochondrial, nuclear genetic, and environmental factors are also known to trigger LHON.Footnote6 Around one in three cases also appears to be sporadic with no definitive family history.Footnote7 Other rare mtDNA mutations account for a further ~5% of the LHON cases.Footnote8 In addition, a second form of LHON mutation in a nuclear encoded gene, DNAJC30, has also been established—described as an autosomal recessive mode of inheritance for LHON (arLHON).Footnote8
Membrane complexes in the mitochondrial respiratory chainsFootnote4

ADP, adenosine diphosphate; ATP, adenosine triphosphate; CyC, cytochrome C; LHON, Leber’s hereditary optic neuropathy; NAD+/NADH, nicotinamide adenine dinucleotide
-
In Europe, the estimated prevalence of LHON with the combined mutations was ~1:45,000.Footnote3 LHON affects predominantly men in their 20s or 30s and is most commonly seen in teenage boys and young men, which account for 80% of the new cases.Footnote1,Footnote7
However, it can occur in individuals of all ages, including children and the elderly, with the age of onset being higher in women.Footnote7 Men are four to five times more likely to be affected than women, but neither gender nor mutational status significantly influences the timing and severity of the initial visual loss.Footnote5 Recent evidence also suggests a protective role of estrogen and could explain LHON vision loss in menopausal women, given the declining levels of estrogen. Footnote9
Who’s at risk

Genetic confirmation of an LHON mutation means everyone on the maternal bloodline is at riskFootnote10

LHON affects both men and womenFootnote5

LHON is most frequently diagnosed in teenagers and young menFootnote7
What LHON eyesight looks like
Optic disc images of 17-year-old boy with LHON from presymptomatic to chronic stageFootnote11
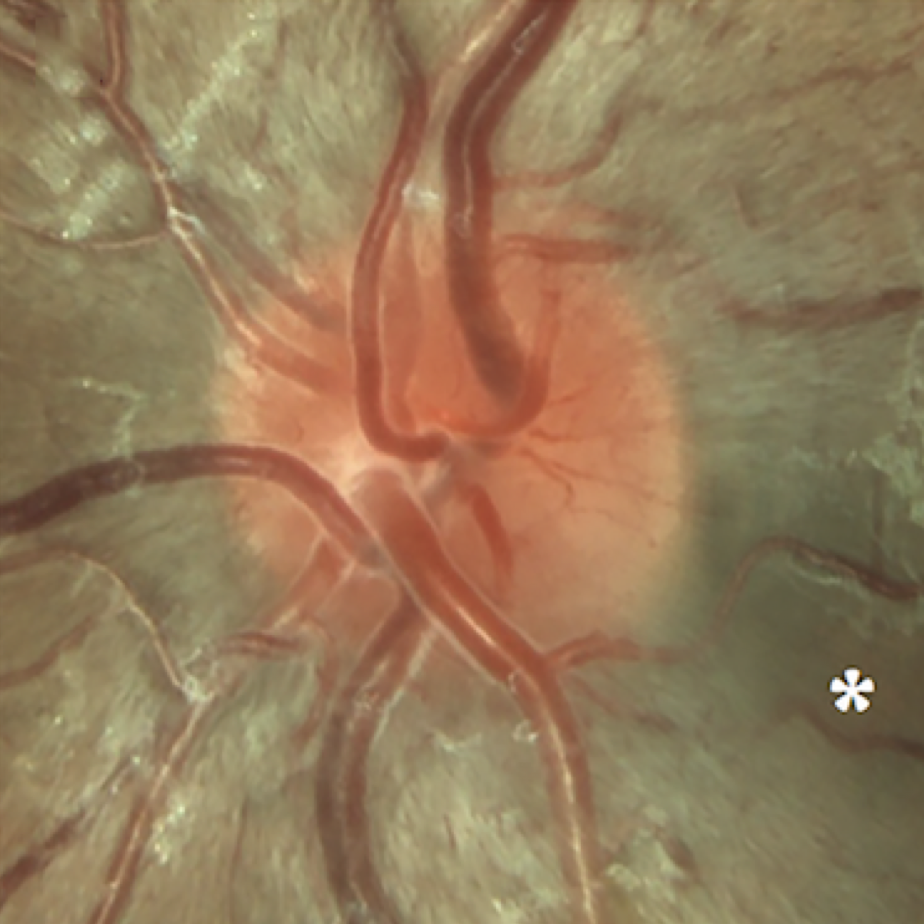
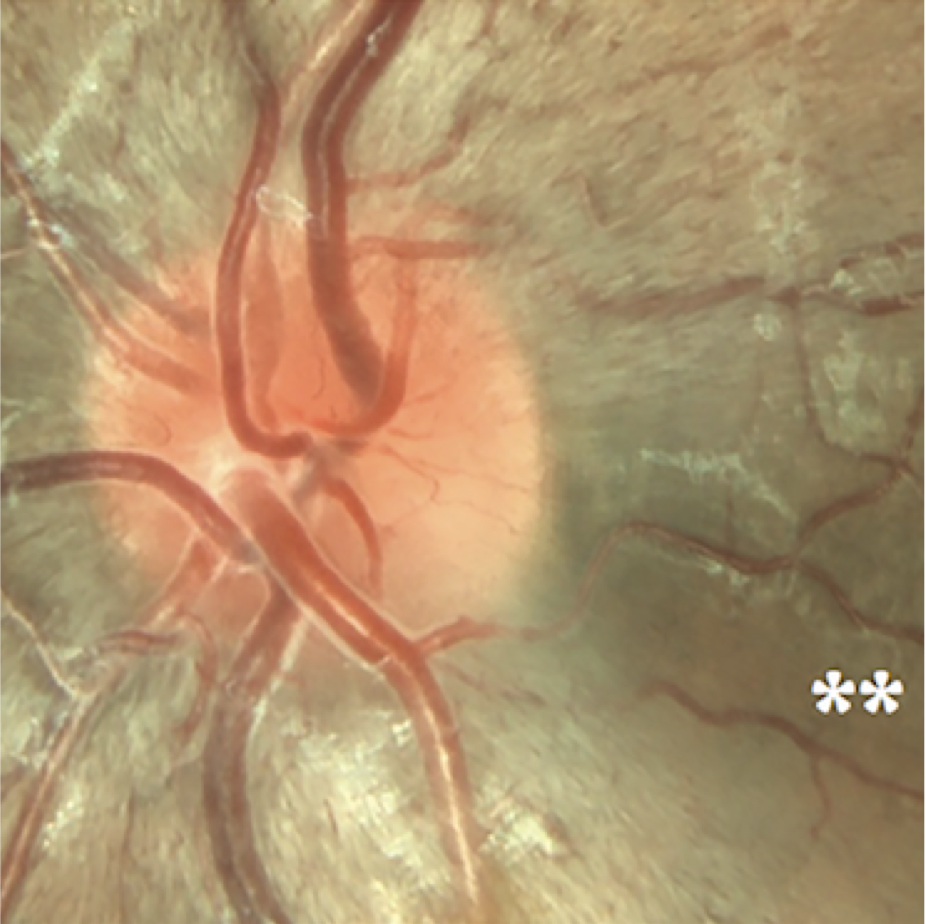
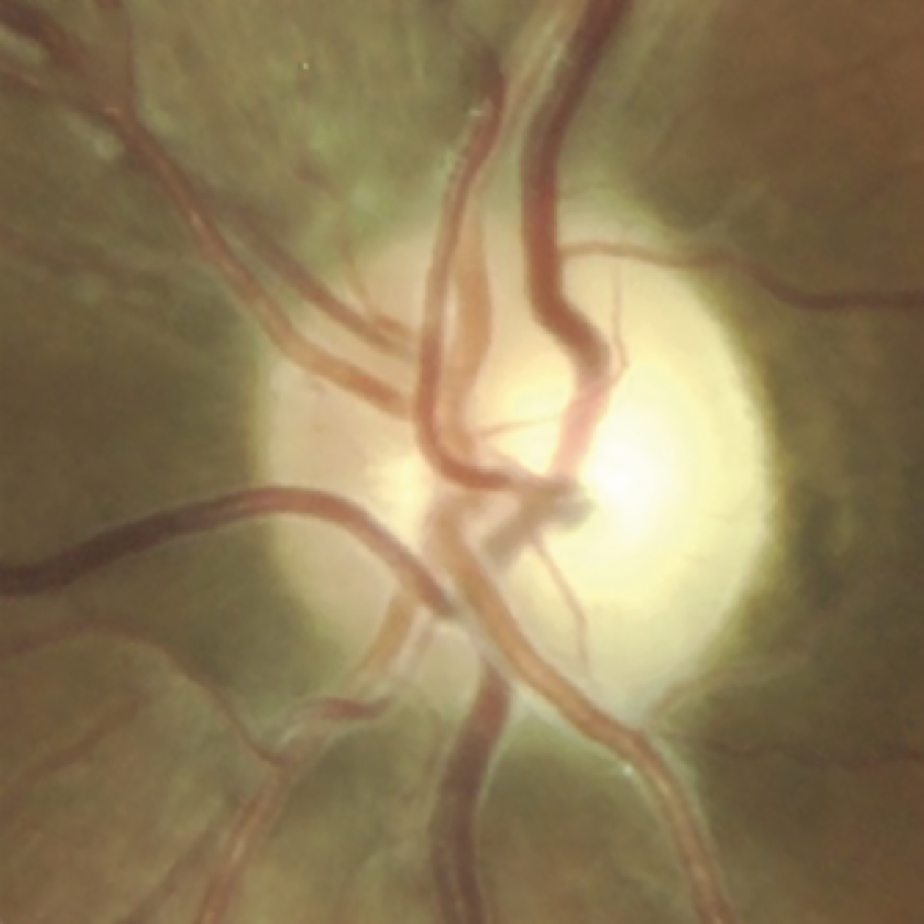
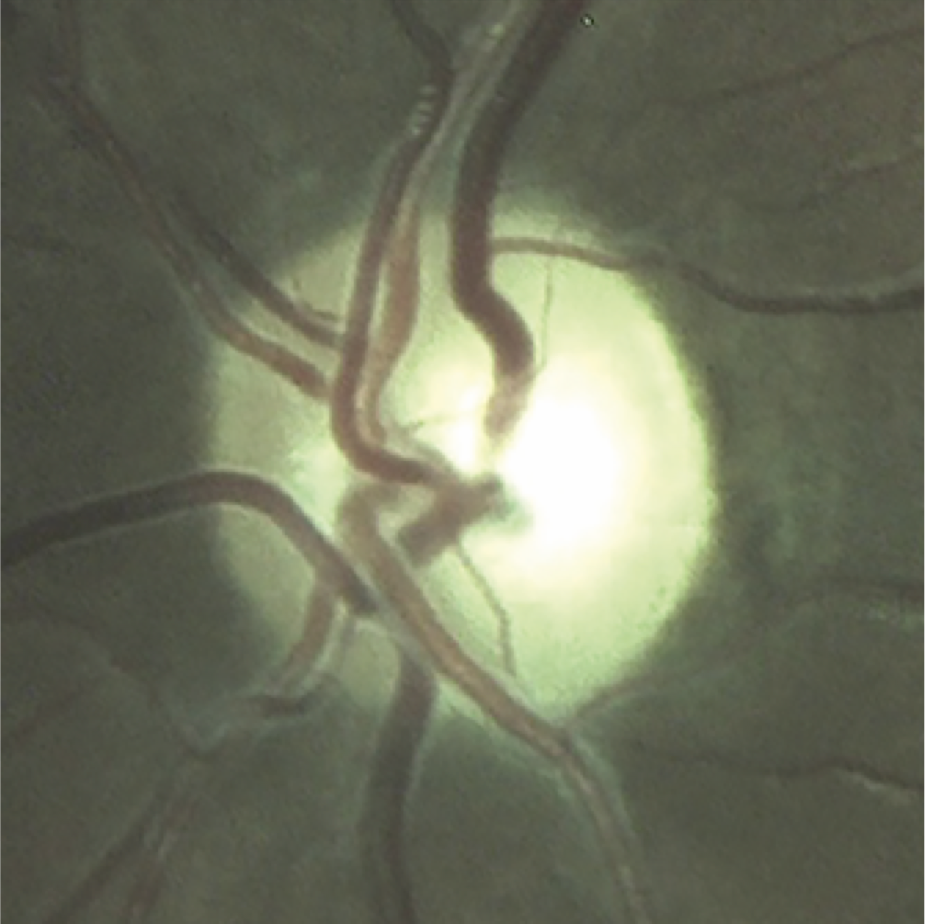
Signs and symptoms
Patients are usually entirely asymptomatic until they develop painless visual blurring affecting the central visual field in one eye (acute phase).Footnote5 Similar symptoms appear in the other eye within weeks or months, with at least 97% of affected individuals having bilateral involvement within one year.Footnote5 Unilateral LHON is very rare.Footnote7 It is important to know that patients sometimes experience nadir (low point in vision) even during treatment, but this should not be a reason to stop the treatment.Footnote4 In some cases, after an initial deterioration of visual acuity and reaching nadir, patients have achieved a clinically relevant recovery after treatment maintenance.Footnote4
LHON red flags
- Bilateral, painless subacute visual failure that develops during young adult lifeFootnote5
- No periocular pain, no pain at eye mobilisationFootnote5
- Disc hyperaemia, oedema of the peripapillary retinal nerve fibre layer, retinal telangiectasia, and increased vascular tortuosityFootnote5
- ~20% of affected individuals show no fundal abnormalities in the acute stage
- Optic disc atrophyFootnote5
- Green-red dyschromatopsiaFootnote5,Footnote10
- Optic nerve dysfunction and absence of other retinal disease alterations on electrophysiologic studiesFootnote5
- Pupillary reflexes are preserved in the early phase (6–12 months) and patients usually report no pain on eye movementFootnote4,Footnote10
Potential risk factors and triggers
A number of environmental factors are also known to trigger LHON in unaffected carriers.Footnote12 Advise patients about avoiding potentially preventable lifestyle risk factors for vision loss:Footnote12,Footnote13
Risk factors
Further triggers of LHON may include:
-
Theodorou-Kanakari A, et al. Adv Ther. 2018;35:1510–18.
Kirches E, et al. Curr Genomics. 2011;12:44–54.
Carelli V, et al. Hum Mol Genet. 2017;26:139–50.
Carelli V, et al. Eur Ophthalmic Rev. 2019;13(Suppl 2).
Ghelli A, et al. J Biol Che. 2003:278(6):4145–50.
Karaarslan C. Adv Ther. 2019:36;3299–307.
Stenton SL, et al. J Clin Invest. 2021:15;131(6):e138267.
Yu-Wai-Man P and Chinnery PF. Leber Hereditary Optic Neuropathy. 2000.
Asanad S, et al. J Curr Ophthalmol. 2019:251–53.
Fraser JA, et al. Surv Ophthalmol. 2010; 55:299–334.
Atlas of Leber’s Hereditary Optic Neuropathy, 2018, MEDonline, ISBN: 978-90-828166-0-0
Sadun A, et al. Curr Treat Options Neurol. 2011:109–17.
Yu-Wai-Man P, et al. Prog Retin Eye Res. 2011;30:81–114.
Disclaimer: The information on this website is intended only to provide knowledge of Leber’s hereditary optic neuropathy (LHON). This information should not be used in place of advice from your GP or other healthcare professional. If in doubt, please contact your doctor for advice. This website has been produced by Chiesi Pharmaceuticals. The website has been developed in accordance with industry and legal standards to provide information for healthcare professionals and the general public about LHON. Chiesi Pharmaceuticals makes every reasonable effort to include accurate and current information. However, the information provided in this website is not exhaustive.
Patient & Caregiver: In case you need to report an adverse drug reaction, please refer to your physician, asking him to fill in and submit the relevant case report to the concerned Health Authority, according to the Pharmacovigilance requirements in force in your Country. Nevertheless, please be kindly reminded that each patient can report any such cases directly to the national reporting system.
Healthcare Professional: In case you want to report an adverse drug reaction you become aware of, please report it to your Health Authority according to the requirements set by the pharmacovigilance legislation.
 Global
Global  Sweden
Sweden  Greece
Greece  Norway
Norway 